ACT Science Practice Test 26
Bộ sưu tập: Tuyển Tập Bộ Đề Thi Đại Học Hoa Kỳ (ACT) - Có Đáp Án Chi Tiết
Số câu hỏi: 23 câuSố mã đề: 1 đềThời gian: 1 giờ
201,787 lượt xem 15,519 lượt làm bài
Bạn chưa làm đề thi này!
Every time a lightbulb is switched on, an electrical circuit is formed. When plugged into outlets that provide a certain voltage, current begins to flow through the bulbs. Current depends on the resistance of the bulb and the voltage of the power supply. The power output of a bulb is a measure of the amount of energy the bulb requires each second. Power is calculated by multiplying current and voltage. Table 9.3 contains data that relate these variables for a basic circuit consisting of one lightbulb and a power supply.
TABLE 9.3
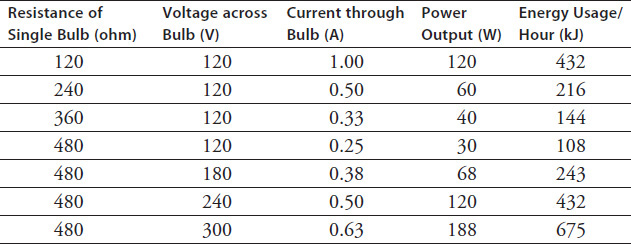
Figure 9.2 shows three configurations of bulbs. When the bulbs are connected in series, they form one path to the power supply. If any bulb in the pathway breaks, all the lights go out because the circuit is no longer complete. In contrast, bulbs connected in parallel are all independently connected to the power supply-in essence, forming their own circuits. Bulbs wired in parallel across a power supply continue to work even when one bulb goes out because each branch forms an independent circuit.
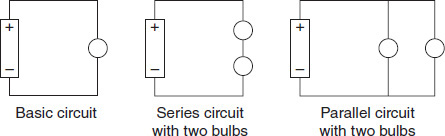
Figure 9.2
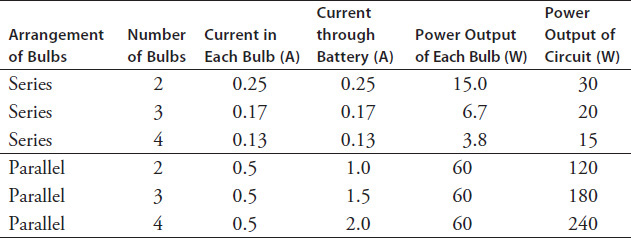
Table 9.4 shows how the number of bulbs in series and parallel affect the current and power values. It gives data for 240-ohm bulbs connected to a 120-V power supply.
TABLE 9.4
Based on the data in Table 9.3, describe the relationship between current, voltage, and resistance.
Current is the ratio of voltage to resistance.
Current is the product of voltage and resistance.
Current is the ratio of resistance to voltage.
Current is proportional to the square of voltage and independent of resistance.
Using information provided in the passage, determine the power output of a 50.0-ohm bulb connected to a 150-V socket that has 3.0 A flowing through it.
3 W
50 W
150 W
450 W
For a fixed voltage, what happens to the power output of a bulb when its resistance triples?
The power triples.
The power increases by a factor of 9.
The power decreases to one-third of its value.
The power decreases to one-ninth of its value.
For a bulb with a given resistance, what happens to the flow of current through the bulb when the voltage of the power supply doubles?
The current doubles.
The current quadruples.
The current decreases to one-half its value.
The current decreases to one-fourth its value.
A 480-ohm bulb is screwed into a 120-V socket. How much energy does it need to stay lit for four hours?
108 kJ
120 kJ
216 kJ
432 kJ
Predict the power required to operate a 600-ohm light bulb when it is plugged into a 120-V outlet.
20 W
24 W
30 W
120 W
According to Tables 9.3 and 9.4, when a fifth bulb is added to a parallel circuit, each bulb will:
output the same amount of energy per second as a bulb in a basic circuit.
output more energy per second than a bulb in a basic circuit.
output less energy per second than a bulb in a basic circuit.
make the other bulbs get brighter.
When a fifth bulb is added to a series circuit, how will the bulb's power output compare to that of a bulb in a four-bulb series circuit?
It will produce the same amount of power.
It will produce twice the power.
It will produce less power.
It will produce no power.
According to the information in Tables 9.3 and 9.4, adding an additional bulb to a parallel circuit:
increases the circuit's power output by decreasing the total resistance of the entire circuit.
decreases the circuit's power output by decreasing the total resistance of the entire circuit.
increases the circuit's power output by increasing the total resistance of the entire circuit.
decreases the circuit's power output by increasing the total resistance of the entire circuit.
The circuit in Figure 9.3 shows Bulbs 2 and 3 wired in parallel. That combination is wired in series with Bulb 1 and the battery. Which of the following statements is FALSE?
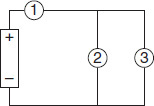
Figure 9.3
If Bulb 1 breaks, the other two bulbs will go out.
If Bulb 2 breaks, the other two bulbs will go out.
If both Bulbs 2 and 3 break, Bulb 1 will go out.
If Bulb 3 breaks, the other bulbs will stay lit.
As bulbs are added to a parallel circuit:
more current flows through each bulb.
less current flows through each bulb.
less power is output from the circuit.
more current flows through the battery.
A child noticed that five bulbs in her electric toy went out simultaneously, but four other bulbs remained lit. What is the most likely circuit arrangement in the toy?
The five bulbs that went out are wired in parallel.
Each of the five bulbs that went out are broken.
The five bulbs that went out are wired in series.
All nine bulbs are wired in parallel.
Adding additional bulbs to a series circuit:
increases the resistance of the entire circuit.
decreases the resistance of the entire circuit.
decreases the power output of the entire circuit.
increases the flow of current in each bulb.
A watt (W) of power is the total joules (J) of electrical energy transferred by a circuit element each second. Given a circuit with three 240-ohm bulbs wired in series to a 120-V power supply, how much energy is transferred by the circuit if it operates for 10 seconds?
6.7 J
67 J
150 J
200 J
How much power does a parallel circuit require if it has six 240-ohm resistors connected to a 120-V power supply?
60 W
120 W
360 W
1,440 W
Since ancient times, scientists, philosophers, and other thinkers considered the smallest pieces of matter to be tiny spherical structures called atoms that were stacked in various arrangements. The ancient Greek philosopher Democritus also suggested that these atoms were indivisible and indestructible. The turn of the twentieth century brought with it a renewed interest in exploring atoms. Two ground-breaking experiments challenged the idea that atoms are the smallest constituent of matter. These experiments were very different, but both pointed to the fact that atoms contained even smaller parts.
Experiment 1: The 1897 Cathode Ray Tube Experiments
Scientists in the late 1890s investigated a curious new device known as a cathode ray tube (CRT). The CRT consisted of a glass tube with a metal wire coming out of each end. When all of the air was removed from the tube and a voltage was applied across the wires, mysterious green rays appeared at one end of the tube. These rays were called cathode rays. It was not immediately known if these rays were a type of wave or a type of particle, but several experiments were conducted on them. First it was discovered that the rays would always be attracted to an area of excess positive charge and away from an area of excess negative charge. Precise measurements could not determine the exact mass of the cathode rays, but it was determined that they did have mass. After that discovery, the rays were considered a particle instead of a wave.
The mass-to-charge ratio of the cathode rays was determined to be more than 1,000 times smaller than the same ratio for any known atom or ion. After subsequent experiments, researchers determined that cathode rays were small particles that broke off of an atom when a voltage was applied.
Experiment 2: The 1911 Gold Foil Experiment
At the time, the accepted model of the atom was a relatively solid sphere, similar to a ball of chocolate chip cookie dough. An experiment in 1911 brought that model into question. A beam of alpha particles (small, high-energy, positively charged particles) was shot into a piece of gold foil approximately 8.6 × 10-8 m in thickness. The alpha particles were thought to have enough energy to pass straight through the foil and hit a detector on the other side, and most of the particles did just that. However, a small fraction of alpha particles were deflected a few degrees as they passed through the foil. Upon closer examination of the data, a more startling fact was found-some alpha particles never hit the detector.
More detectors were added around the gold foil, and it was discovered that a tiny portion of the alpha particles, 1 out of every 20,000 particles, was deflected 90 degrees or more from the beam. Some particles even bounced straight back toward the alpha particle source. The scientist was so surprised by the results that he stated, "It was as if you fired a 15-inch shell at a sheet of tissue paper and it came back to you." It was concluded that there must be some particle inside an atom causing these major deflections of alpha particles.
Which of the following best describes what each experiment concluded about the newly discovered small particles that make up atoms?
Experiment 1, Experiment 2
Dense and sturdy, Positively charged
Negatively charged, Dense
Positively charged, Negatively charged
Small, Low energy
Which of the following is the best conclusion concerning why the cathode rays in Experiment 1 had a mass-to-charge ratio 1,000 times smaller than that of any known atom?
Cathode ray particles are 1,000 times less massive than any known atom.
Cathode ray particles have 1,000 times less charge than any known atom.
Cathode ray particles have 1,000 times more charge than any known atom.
Cathode ray particles could either be less massive or have a greater charge than any known atom.
Modern chemistry books discuss several subatomic particles. Figure 9.4 offers a common diagram of such particles. The symbol (-) means negative, (+) means positive, and (ø) means neutral. Which particle would have an undefined mass-to-charge ratio?
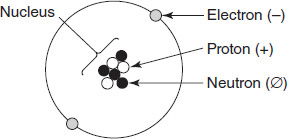
Figure 9.4
Electron
Proton
Neutron
Nucleus
Which of the following statements do the data from both experiments support?
Atoms are highly charged particles.
Atoms are composed of a dense core known as a nucleus.
Atoms are not indestructible and indivisible.
The mass-to-charge ratio of atoms is much smaller than was originally thought.
Atoms, once thought to be solid spheres, have instead been proven to be mostly empty space with just a few tiny particles giving each atom its properties. Which data from either Experiment 1 or Experiment 2 best explains this fact?
Experiment 1: Cathode rays are attracted to areas of positive electric charge.
Experiment 1: Cathode rays have a small mass-to-charge ratio.
Experiment 2: Some alpha particles were deflected from the beam at wide angles.
Experiment 2: One out of every 20,000 particles got deflected to a large extent.
A micrometer (μm) is a common unit to measure small objects. One micrometer is equivalent to 0.000001 m. How thick was the gold foil in Experiment 2?
8,600 μm
86 μm
0.86 μm
0.086 μm
Which scientists would be most likely to write the following in their lab notebook: "I can see no escape from the conclusion that they are charges of negative electricity carried by particles of matter"?
Scientists working on Experiment 1
Scientists working on Experiment 2
Scientists working on either experiment
Scientists working on both experiments
Which headline best matches the conclusions of the experiment?
Gold Foil: Alpha Particles Are Found Inside Atoms
Cathode Rays Destroy Atoms
Gold Foil: Solid Particle in Atom Incredibly Small
Cathode Rays: Most Go Through But a Few Bounce Off
1 mã đề 55 câu hỏi
1 mã đề 23 câu hỏi
1 mã đề 10 câu hỏi
1 mã đề 24 câu hỏi
1 mã đề 22 câu hỏi

